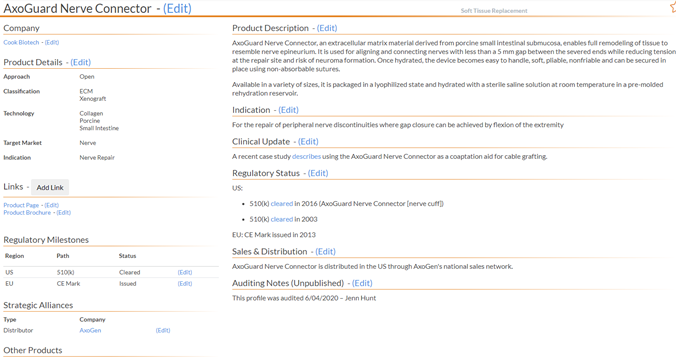
5.1 Product Profile Overview
5.01 Creating a Product Profile - Left Side - to create a product profile, click the "Add Product" button.
5.02 Product Profile - Left Side - click a product link from the GPS module to edit a product profile.
The left side of the page will have:
Product Details - if one is missing, alert the analyst.
Approach
Classification
Technology
Target Market
Indication
Links - this will list the Product Page at a minimum, add more if you can find appropriate links. If links are already listed, you can reorder them by dragging the link title up or down to the appropriate placement.
Product Page
Product Brochure
Surgical Technique
IFU
etc.
Regulatory Milestones - The US should be listed first. Please reference the Clinical/Regulatory and Regulatory Milestones sections for details. What appears in this section of the product profile needs to match what is contained in the "Regulatory Status" article.
Strategic Alliance- Not all product profiles will have this section. Please refer to the Strategic Alliance section for details. Any strategic alliances added here should also be added to the Company Overview and Module Overview pages.
Other Products - If there are more products listed in the module and/or product category they will appear in this section. An example would be: a product within the Extremities module of Company ABC. Not all product profiles have this section.
5.12 Product Profile - Right Side The right side of the page will have:
Product Description (required) - The analyst will input a detailed product description. Check for spelling errors, proper product name spelling and capitalization, spacing and grammatical errors. Please refer to the Product Description page for more details.
Indication (required) - The analyst will describe how the product should be used. This section might not end with a period depending on how the description is written.
Clinical Update (not required) - Only studies published on clinicaltrials.gov should be listed here. If there are no clinical updates, please write "No recent clinical updates". If there are multiple studies keep the singular of Clinical Update as the title.
Clinical Trial Results (not required) - Under the Product Profile Clinical Update section, analysts may link to clinical trial results there if those results are reported on clinicaltrials.gov. If it is published in a journal, it should be placed under Published Studies for that product.
Published Studies (not required) - Studies that are not published on clinicaltrials.gov belong in this section. It's the catch all for all other published studies. If there is only one study keep the plural of Published Studies as the title.
Regulatory Status (required) - This should list the Regulatory Milestones that you see on the left side. The US should be first. If there are more than one milestone in a country, use bullet points to list them. Please reference Clinical/Regulatory section for details.
Sales and Distribution (not required) - This section will detail how and where this product is sold and distributed.
Auditing Notes - This section you add when you have QC'd this product profile. Click Content at the top of the page, click Add Content, type Auditing Notes in the Content Title, select Edit in the Status drop down and type - This profile was audited on mm/dd/yyyy - First Name Last Name