5.0 Product Profiles
5.5 Regulatory Milestones
5.51 The US should be listed first. Please reference the Clinical/Regulatory section for details.
5.52 The Regulatory Milestones region, path and status should match the Regulatory Status section. The Regulatory Status section might have more details such as a date or small description if there is more than one in a region.
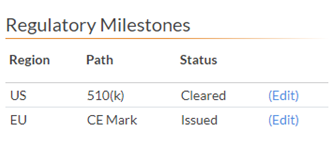
| US Regulatory | ||
| Region | Path | Status |
| US | 510 (k) | Cleared |
| US | Class I | Exempt |
| OUS Regulatory | ||
| Region | Path | Status |
| Argentina | ANMAT | Approved |
| Australia | TGA | Approved |
| Brazil | ANVISA | Approved |
| Canada | Health Canada | Issued |
| Chile | ISP | Approved |
| China | NMPA | Approved |
| EU | CE Mark | Issued |
| EU | EMEA | Clinicals |
| India | CE Mark | Issued |
| India | Genetic Engineering Approval Committee | Approved |
| India | New Delhi and State Drug Control Administration | Approved |
| India | Ministry of Environmental and Forests | Approved |
| India | DCGI | Approved |
| India | CDSCO | Approved |
| Israel | Israel Ministry of Health | Approved |
| Japan | MHLW | Approved |
| Mexico | ||
| Netherlands | Human Tissue | On Market |
| Netherlands | ISO Certification | Issued |
| New Zealand | WAND | Registered |
| New Zealand | Medsafe | Approved |
| Singapore | Heath Sciences Authority | Registered/Approved |
| South Korea | MFDS | Approved |
| Sweden | Medical Products Agency | Approved |
| Taiwan | Taiwan DOH | Approved |
| Thailand | Thai FDA | Cleared |
| UK | 510 (k) | Cleared |
| UK | CE Mark | Issued |
